Advancing Precision Medicine through Precompetitive Collaborations and Large-Scale AI Models
Sean Khozin, MD, MPH & Jon McDunn, PhD
The integration of artificial intelligence (AI) into the life sciences industry is providing new avenues for reshaping existing drug discovery and development practices. While the adoption of AI in biomedicine is still in its early stages, two distinct approaches are emerging: targeted AI applications and large-scale model development efforts.
Targeted AI Applications: Streamlining Drug Development Processes
Targeted AI applications primarily focus on optimizing specific, process-oriented aspects of drug development programs in the short term. These applications aim to streamline bottlenecks and improve efficiency in various stages of the drug development pipeline. For instance, established companies are leveraging AI algorithms to optimize patient recruitment and selection in clinical trials, identify potential safety signals, and predict patient dropout rates. While these targeted applications can help reduce the time, cost, and risk associated with drug development and improve overall efficiency, they are ultimately limited in their ability to drive fundamental changes in our understanding of disease biology and mechanisms.
Large-Scale AI Models: Unveiling Novel Insights into Disease Mechanisms
In contrast to targeted AI applications, large-scale models have the potential to advance drug discovery and clinical development by utilizing multimodal, diverse datasets to elucidate novel insights into disease mechanisms, identify new therapeutic targets, and facilitate the development and validation of biomarkers and intermediate endpoints. These models can provide a more comprehensive understanding of the complex biological processes underlying disease pathophysiology, enabling the identification of previously unknown pathways and potential targets for intervention. However, the development of truly transformative models requires access to extensive, diverse, and well-curated datasets, as well as the expertise and computational resources necessary to train and validate these models. Individual companies often lack the scale, diversity, and quality of data required to develop such models independently. Moreover, the creation of transformative AI models often necessitates cross-disciplinary collaboration and knowledge sharing that can extend beyond the core objectives of single organizations.
Precompetitive Collaborations: Overcoming Limitations and Fostering Innovation
Precompetitive collaborations provide a framework for overcoming the limitations associated with the development of truly transformative AI models. By pooling resources, expertise, and data from multiple organizations, precompetitive collaborations can create the robust, diverse datasets that are essential for training sophisticated AI models. These collaborations foster critical partnerships among life sciences companies, academic institutions, and technology providers, facilitating the exchange of knowledge and best practices across disciplines.
Project Data Sphere: A Prime Example of Precompetitive Collaboration
Project Data Sphere, a nonprofit initiative of the CEO Roundtable on Cancer, exemplifies the potential for precompetitive collaboration to drive progress in oncology research and care. Two key initiatives within Project Data Sphere, namely autoRECIST and the Immune-Related Adverse Events (irAEs) program, illustrate how the collaboration of stakeholders from academic institutions, regulators, and industry partners can effectively address critical challenges in the field of oncology.
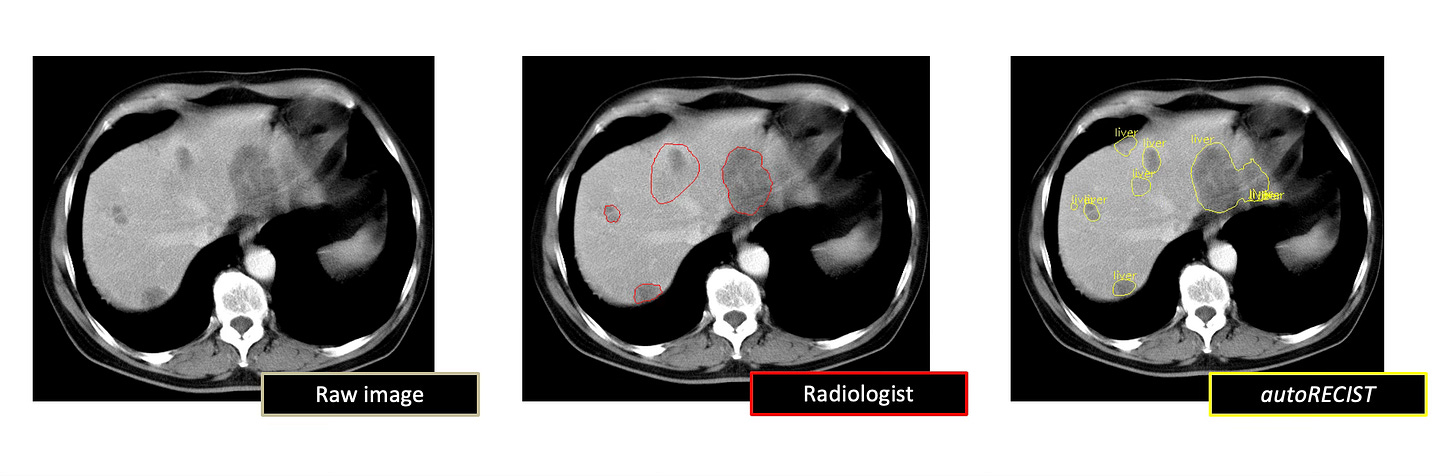
The autoRECIST initiative aims to transform the assessment of tumor burden and treatment response evaluation in oncology clinical trials by leveraging advanced AI techniques. The project focuses on developing deep learning algorithms to automate the Response Evaluation Criteria in Solid Tumors (RECIST) assessment process, which has the potential to significantly improve the accuracy, consistency, and efficiency of tumor response assessments. By automating RECIST calculations, the initiative seeks to address the limitations of manual tumor assessment driven by human visual inspection, such as the 30% discordance in radiological evaluation of tumor-based endpoints between primary investigator and independent radiologist reviews in registrational studies. Furthermore, Project Data Sphere aims to build upon the foundation of autoRECIST to develop autonomous AI agents for the evaluation of tumor burden and dynamics based on DICOM imaging, which can provide a more comprehensive and nuanced assessment of tumor response to treatment.
Concurrently, the irAEs program within Project Data Sphere focuses on addressing the significant challenge of immune-related adverse events in cancer immunotherapy. By collaborating with leading academic centers, the US Food and Drug Administration, and leveraging real-world data, the initiative aims to enhance the diagnosis, management, and prevention of irAEs, which can cause significant morbidity in patients receiving immunotherapies such as immune checkpoint inhibitors (ICIs). The program employs translational research approaches to uncover new biomarkers for serious irAEs, which can inform the development of personalized treatment strategies that minimize the risk of adverse events while maximizing the therapeutic benefits of immunotherapy.
The precompetitive nature of these initiatives has been crucial to their success, as it has ensured that the benefits of the research are disseminated across the entire cancer community. By pooling intellectual resources, expertise, and data from multiple stakeholders, Project Data Sphere and its collaborators are developing robust, validated, and generalizable solutions that accelerate innovation, improve the efficiency of clinical research, and ultimately provide patients with faster access to life-saving treatments. Furthermore, the secondary use of clinical research data in these collaborations honors patient contributions to science, as surveys indicate that a substantial number of patients who participate in clinical research desire their data to be used broadly.
Driving Transformative Innovation through Strategic Collaborations
As the biomedical research industry continues to evolve, organizations that engage in strategically designed precompetitive collaborations will be best positioned to drive transformative innovation and shape the future of drug development. The success of these collaborations in developing transformative AI models depends on effective governance structures, clear objectives, and equitable benefit-sharing among participants. Collaborators must establish trust, transparency, and accountability to foster an environment conducive to innovation and knowledge sharing. Moreover, collaborations should be designed to align with the strategic priorities of participating organizations, ensuring that the outcomes of the collaboration are relevant and valuable to all stakeholders.
By harnessing the power of AI and collective expertise, these collaborative efforts promise to revolutionize our understanding of disease biology, identify novel therapeutic targets, and ultimately improve patient outcomes in the ongoing battle against cancer and other complex diseases. As we move forward, it is essential to recognize the complementary roles of targeted AI applications and transformative large-scale model development efforts in advancing the frontiers of science for the benefit of patients worldwide.


